Helping to significantly improve global health outcomes – but most importantly, save lives.

What We Do
BG Research was established as an innovation development and manufacturing pipeline to our partner company BioGene.
We conceptualise, develop and manufacture pioneering molecular and engineering technologies with the aim of helping to significantly improving global health outcomes – but most importantly save lives.
Our Capabilities
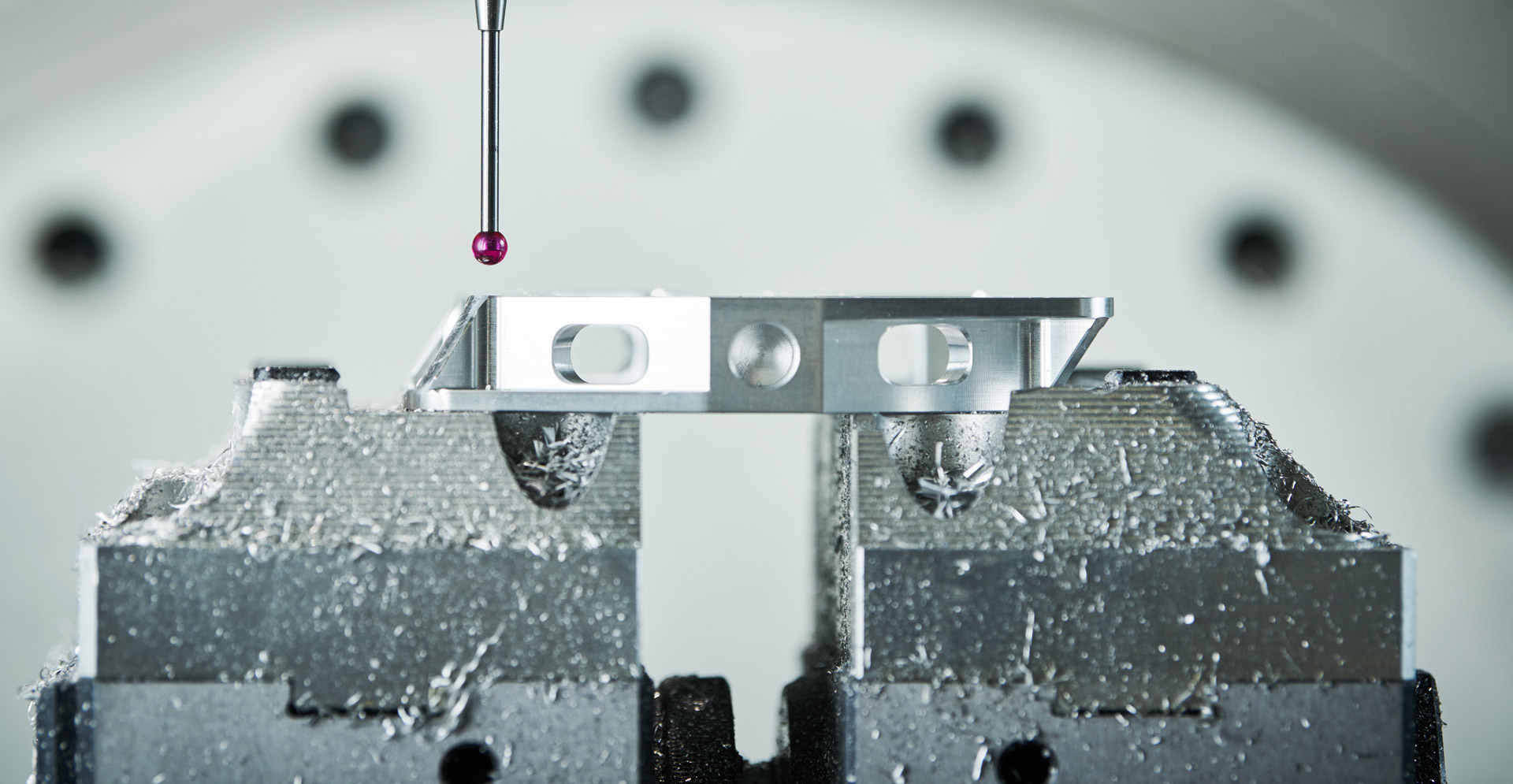
With over 25 years of experience our in-house multi-disciplinary team specialises in identifying and implementing opportunities for improvement in molecular diagnostics through the creation of bespoke and novel technological solutions to improve global health outcomes. We have capabilities in all key areas to suit a broad scope of applications and requirements; even the most complex and challenging of ones.
At Patient Testing
At Patient Testing (APT) represents a paradigm shift in molecular diagnostics. This novel, technology led actionable strategy focuses on testing whilst the patient (human or animal) waits in a decentralised environment (no specialist lab facilities or infrastructure required) by users with minimal training.
Patients can be rapidly diagnosed with the sensitivity to enable the early detection of subclinical, asymptomatic and non-symptomatic cases. This unique strategy provides actionable results and data in ‘real time’ to assist in triaging, treatment, and surveillance activities to help break onward disease transmission chains and mitigate the effects of High Consequence Infectious Diseases (HCIDs) on society.
Everything we do and our continuous research into new innovations is dedicated to supporting At Patient Testing. APT represents true point of care testing in any environment without frontiers.